Introduction: The Invisible Force That Shapes Our Weather
Imagine standing at the bottom of an immense ocean, feeling the weight of countless gallons of water pressing down upon you. Now, realize that we all live in a similar situation every day, not under water, but under a vast sea of air. This invisible ocean of atmosphere, stretching from the ground to the edge of space, exerts a constant pressure on everything it touches. This pressure, known as atmospheric pressure or air pressure, is a fundamental component of our weather and climate. To measure and understand this crucial force, we turn to an ingenious device: the barometer.
The Birth of the Barometer: A Scientific Revolution
The story of the barometer begins in 17th century Italy, during a time of great scientific awakening. In 1643, Evangelista Torricelli, a student of the famed Galileo Galilei, conducted an experiment that would change our understanding of the atmosphere forever. Torricelli filled a long glass tube with mercury, sealed one end, and inverted it into a bowl of mercury. To his amazement, the mercury in the tube fell, leaving a space at the top – the world's first artificially created vacuum.
This simple yet profound experiment demonstrated that air has weight and exerts pressure. The height of the mercury column in the tube varied with changes in atmospheric pressure, providing a way to measure this previously invisible force. Torricelli had invented the first barometer, a device that would become instrumental in weather prediction and our understanding of atmospheric science.
The Inner Workings of Barometers: From Mercury to Silicon
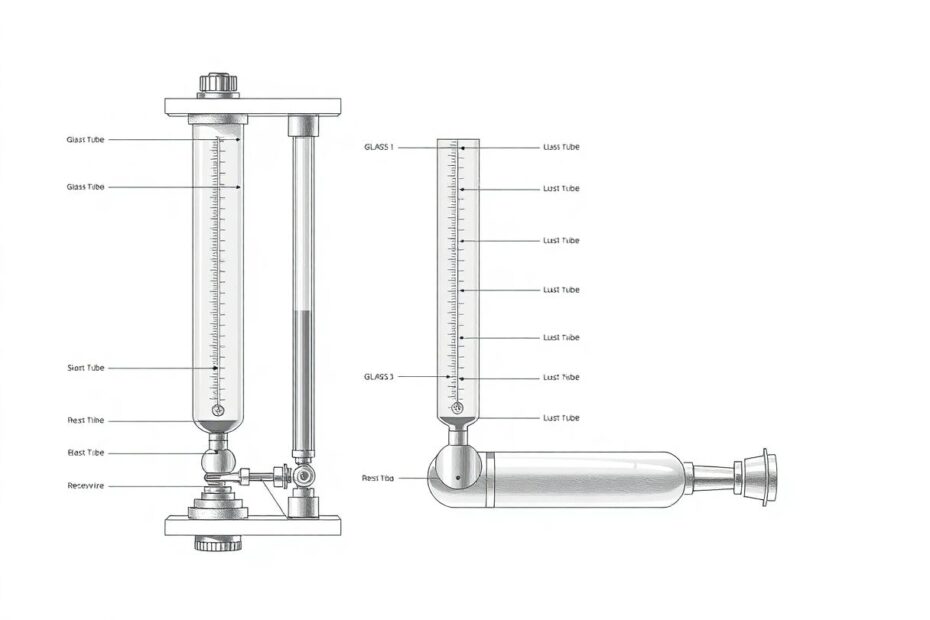
The Classic Mercury Barometer
Torricelli's original design, known as the mercury barometer, remains a standard in meteorological stations worldwide. Its operation is elegantly simple:
A glass tube, typically about 85 cm long and sealed at one end, is filled with mercury and inverted into a reservoir of mercury. The weight of the atmosphere pressing down on the mercury in the reservoir supports the column of mercury in the tube. As air pressure changes, the height of the mercury column rises or falls. At sea level, under standard conditions, this column is about 760 mm (29.92 inches) high.
The use of mercury is crucial due to its high density. If water were used instead, the barometer would need to be over 10 meters tall! However, the toxicity of mercury has led to the development of safer alternatives for everyday use.
The Aneroid Barometer: Pressure Without Liquids
In 1844, French scientist Lucien Vidi invented the aneroid barometer, which operates without any liquid. The heart of this device is a small, flexible metal box called an aneroid cell. This cell is partially evacuated of air and sealed. As atmospheric pressure changes, the cell expands or contracts slightly.
This tiny movement is amplified through a system of levers and springs, moving a needle across a calibrated dial. Modern aneroid barometers can be remarkably accurate and are widely used in both scientific and household applications.
Electronic Barometers: The Digital Revolution
The advent of microelectronics has ushered in a new era of barometric measurement. Modern electronic barometers use pressure-sensitive components, often made of silicon, that deform under changing air pressure. This deformation alters the electrical properties of the sensor, which can be measured and converted into a digital pressure reading.
These digital barometers are now ubiquitous, found in weather stations, smartphones, and even some wristwatches. They offer high accuracy, rapid response times, and the ability to log data over time, making them invaluable tools for both professional meteorologists and weather enthusiasts.
Understanding Barometric Pressure Readings
Barometric pressure can be expressed in various units, reflecting its long history and wide range of applications. Common units include:
- Inches of mercury (inHg): Used primarily in the United States for weather reports.
- Millimeters of mercury (mmHg): Often used in medical contexts.
- Hectopascals (hPa) or millibars (mb): The standard unit in meteorology.
- Pascals (Pa) or kilopascals (kPa): The SI unit of pressure.
Standard atmospheric pressure at sea level is defined as 1013.25 hPa (or mb), which is equivalent to 29.92 inHg, 760 mmHg, or 101.325 kPa. However, actual pressure varies constantly based on weather conditions and altitude.
Barometers and Weather Forecasting: Reading the Atmospheric Tea Leaves
The ability of barometers to predict weather patterns has made them indispensable tools in meteorology. Generally speaking:
- Rising pressure often indicates improving weather, with clear, sunny skies on the horizon.
- Falling pressure frequently signals the approach of storms or precipitation.
- Steady pressure usually suggests stable weather conditions.
However, it's important to note that barometer readings alone aren't sufficient for accurate weather prediction. Modern forecasting relies on a complex interplay of various measurements, satellite data, and sophisticated computer models. Nonetheless, the barometer remains a crucial piece of this meteorological puzzle.
Beyond Weather: Specialized Applications of Barometric Measurement
Barographs: Pressure Over Time
A barograph is a specialized type of barometer that continuously records air pressure over time. Traditional barographs use an aneroid barometer mechanism connected to a pen that traces a line on a rotating drum covered with graph paper. This creates a visual record of pressure changes, allowing meteorologists to analyze trends and patterns.
Modern electronic barographs have replaced the mechanical components with digital sensors and computer storage, but the principle remains the same. These devices are invaluable for tracking the movement of weather systems and understanding long-term atmospheric trends.
Altimeters: Barometers that Measure Height
Altimeters, essential instruments in aviation and mountaineering, are essentially specialized barometers. They work on the principle that air pressure decreases predictably with increasing altitude. By measuring the air pressure and comparing it to a known reference (usually sea level pressure), an altimeter can determine the altitude of an aircraft or the height of a mountain.
However, because air pressure can vary due to weather conditions, pilots must regularly calibrate their altimeters to ensure accurate readings. This calibration process underscores the intricate relationship between air pressure, weather, and altitude.
The Impact of Temperature on Barometric Readings
Temperature plays a significant role in barometric measurements, as it affects air density and, consequently, pressure readings. As air warms, it expands and becomes less dense, which can lead to lower pressure readings even if the actual mass of air hasn't changed.
To account for this, high-quality barometers often include temperature compensation mechanisms. In mercury barometers, this might involve a correction factor based on the temperature of the mercury. Electronic barometers can use built-in temperature sensors to automatically adjust readings.
This temperature dependence highlights the complexity of atmospheric dynamics and the importance of considering multiple factors when interpreting barometric data.
DIY Barometers: Understanding Through Hands-on Experience
Creating a simple barometer at home can provide a tangible understanding of how these instruments work. A basic water barometer can be made with everyday materials:
- Fill a glass about two-thirds full of water.
- Place an empty, narrow-necked bottle upside down in the glass, ensuring the bottle's neck is submerged but not touching the bottom of the glass.
- Mark the initial water level in the bottle's neck.
- Observe how the water level changes over time with variations in air pressure.
While not as accurate as commercial instruments, this home experiment illustrates the fundamental principle behind Torricelli's mercury barometer. It demonstrates how changes in air pressure can move a column of liquid, providing a visual representation of atmospheric variations.
The Future of Barometric Measurement: Miniaturization and Integration
As technology advances, barometers continue to evolve. Some key trends include:
Miniaturization: MEMS (Micro-Electro-Mechanical Systems) technology has enabled the creation of tiny barometers that can fit inside smartphones and wearable devices. These microscopic sensors can detect changes in altitude as small as a single stair step.
Integration: Modern weather stations often incorporate barometers alongside other sensors for temperature, humidity, wind speed, and more. This integration provides a comprehensive view of atmospheric conditions.
Networking: Internet-connected barometers contribute to crowdsourced weather data networks, enhancing the resolution and accuracy of weather forecasts and climate models.
Conclusion: The Enduring Significance of Barometers
From Torricelli's mercury-filled tube to the silicon chip in your smartphone, barometers have come a long way in the past four centuries. Yet, their fundamental purpose remains unchanged: to measure the invisible but omnipresent force of atmospheric pressure.
Barometers remind us that we live at the bottom of an ocean of air, subject to its ebbs and flows. They provide a tangible link to the vast, dynamic system of our atmosphere, helping us understand and predict the weather patterns that shape our daily lives.
Whether you're a meteorologist studying global weather systems, a pilot navigating through changing altitudes, or simply someone curious about the world around you, barometers offer a window into the complex, ever-changing blanket of air that surrounds our planet.
The next time you check a weather forecast or glance at a barometer, take a moment to appreciate the centuries of scientific discovery and engineering ingenuity behind this seemingly simple device. It's not just measuring air pressure; it's telling the ongoing story of our atmosphere, one reading at a time.