Have you ever marveled at how a simple thermos can keep your coffee piping hot for hours or maintain the chill of your iced tea on a sweltering day? The secret lies in the ingenious design of the vacuum flask, a marvel of thermal engineering that has been keeping our beverages at the perfect temperature for over a century. In this deep dive, we'll unravel the science behind these remarkable containers and explore how they manage to defy the normal rules of heat transfer.
The Challenge: Battling the Laws of Thermodynamics
To appreciate the genius of the vacuum flask, we must first understand the formidable opponent it faces: the relentless tendency of heat to spread out and equalize. This fundamental principle of thermodynamics means that, left to its own devices, your steaming cup of coffee will inevitably cool to room temperature, while your refreshing cold drink will warm up.
This heat transfer occurs through three primary mechanisms:
- Conduction: The direct transfer of heat through materials in contact with each other.
- Convection: Heat transfer via the movement of fluids or gases.
- Radiation: The emission of heat as electromagnetic waves.
To keep a beverage at its desired temperature, a container must effectively combat all three of these heat transfer methods. This is precisely what a vacuum flask is designed to do.
The Vacuum Flask: A Triumph of Thermal Engineering
The modern vacuum flask, patented in the early 1900s, is a testament to the power of applied physics. Let's break down its key components and examine how each contributes to its temperature-maintaining prowess.
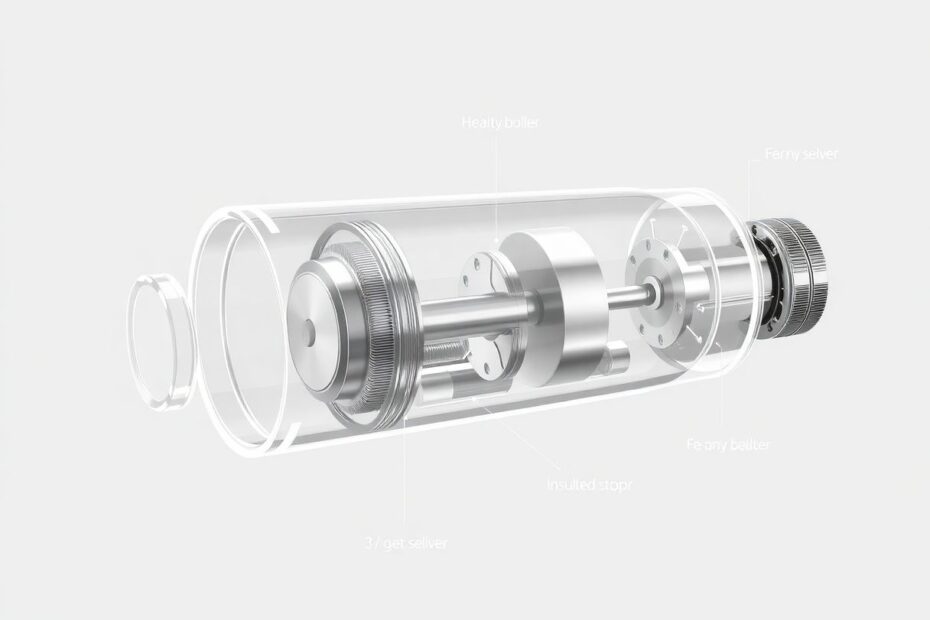
Double-Walled Construction: The First Line of Defense
At its core, a vacuum flask consists of two containers, one nested inside the other. This double-walled construction is the foundation of its insulating capabilities:
- The inner container holds the beverage and is typically made of glass or stainless steel.
- The outer container provides protection and a safe-to-touch exterior.
The Vacuum Layer: Nature's Ultimate Insulator
The space between the two walls is evacuated to create a near-perfect vacuum. This vacuum is the heart of the flask's insulating power:
- By removing almost all molecules from this space, conduction is virtually eliminated. With no medium for heat to travel through, thermal energy is effectively trapped.
- The absence of air or other fluids prevents convection currents from forming, further inhibiting heat transfer.
To put this in perspective, the vacuum in a high-quality thermos can have a pressure as low as 10^-6 torr, which is about one-billionth of atmospheric pressure at sea level. This extreme vacuum is crucial for maximizing insulation.
Reflective Coating: Bouncing Heat Back
The inner wall (and sometimes the outer wall facing the vacuum) is coated with a highly reflective material, usually silver or aluminum. This reflective layer serves a critical function:
- It bounces radiant heat back into the container, further reducing heat loss through radiation.
- The efficiency of this reflection can be as high as 95%, meaning only a tiny fraction of radiant heat manages to escape.
Insulated Stopper: Sealing the Deal
The top of the flask is sealed with an insulated stopper, often made of cork, plastic, or rubber with additional insulating materials:
- This prevents air from entering or leaving the flask, eliminating another potential avenue for heat transfer.
- Some high-end thermoses use multiple layers in the stopper for enhanced insulation.
The Symphony of Insulation: How It All Works Together
Imagine you've just filled your thermos with hot coffee. Here's a step-by-step breakdown of how the flask keeps it hot:
Conduction is minimized:
- The vacuum between the walls prevents direct heat transfer through the sides of the flask.
- Any minimal conduction that occurs at the neck is slowed by the insulated stopper.
Convection is eliminated:
- With no air in the vacuum layer, there's no medium for convection currents to form.
- The tight seal at the top prevents air circulation in and out of the flask.
Radiation is reflected:
- Any heat that does make it to the vacuum layer as infrared radiation is bounced back by the reflective coating.
The result is a remarkably effective insulation system. A high-quality vacuum flask can keep hot liquids above 60°C (140°F) for up to 24 hours, and cold liquids below 10°C (50°F) for a similar duration.
Versatility in Temperature Control: Keeping Things Cold
Interestingly, the same principles that keep hot drinks hot also keep cold drinks cold. The vacuum flask doesn't discriminate between hot and cold – it simply resists temperature change in either direction.
For cold beverages:
- The vacuum and reflective layer prevent heat from the outside environment from warming the contents.
- The insulated stopper prevents warm air from entering and warming the drink.
This bidirectional insulation makes vacuum flasks incredibly versatile, suitable for a wide range of applications beyond just keeping your coffee hot.
Beyond Beverages: Real-World Applications
While we often associate vacuum flasks with our morning coffee or a refreshing drink on a hot day, their applications extend far beyond personal use:
Scientific Research: In laboratories, vacuum flasks (often called Dewar flasks in this context) are crucial for storing and transporting cryogenic liquids like liquid nitrogen. These flasks can maintain temperatures as low as -196°C (-320.8°F) for extended periods.
Medical Field: Vacuum flasks play a vital role in transporting temperature-sensitive medications, vaccines, and biological samples. For instance, some COVID-19 vaccines require ultra-cold storage, and modified vacuum flask technology is used in their transportation.
Food Industry: Large-scale vacuum flasks help in transporting and storing temperature-sensitive foods. This is particularly important in the dairy industry and for transporting perishable goods over long distances.
Space Exploration: Modified vacuum flask technology is used in spacecraft to protect sensitive equipment from extreme temperature fluctuations in space. The insulation principles are also applied in spacesuits to protect astronauts from the extreme cold of space.
Industrial Applications: In many industrial processes, maintaining specific temperatures is crucial. Vacuum insulated piping, based on the same principles as vacuum flasks, is used to transport liquefied gases and other temperature-sensitive materials.
The Limits of Insulation: Understanding Thermal Efficiency
While vacuum flasks are remarkably effective, they're not perfect perpetual temperature maintainers. Some heat transfer still occurs, primarily through:
- The stopper, which isn't a perfect insulator.
- Minimal radiation that makes it through the reflective layer.
- Slight conduction at points where the inner and outer containers meet.
To quantify this, we can look at the thermal efficiency of vacuum flasks. A typical high-quality thermos might have a heat loss rate of about 2-3°C per hour for hot liquids. This means that over a 24-hour period, a drink starting at 90°C might cool to around 40-50°C, still warm but noticeably cooler than its starting temperature.
From Lab to Kitchen: A Brief History of the Vacuum Flask
The vacuum flask as we know it today has a fascinating history that spans over a century:
1892: Sir James Dewar, a Scottish scientist, invents the vacuum flask for his cryogenic experiments. His original design, known as the Dewar flask, was primarily used for scientific purposes.
1904: German glassblowers Reinhold Burger and Albert Aschenbrenner recognize the commercial potential of Dewar's invention. They refine the design and start the Thermos company.
Early 1900s: The "Thermos" brand becomes so popular that it becomes a generic term for vacuum flasks, much like "Kleenex" for tissues.
1907: The first patent for a commercial vacuum flask is granted to Burger and Aschenbrenner.
Mid-20th century: Advancements in materials science lead to the development of more durable stainless steel vacuum flasks.
Interestingly, Dewar never patented his invention, focusing instead on its scientific applications. It was the commercial insight of Burger and Aschenbrenner that brought this technology into everyday life, transforming it from a laboratory curiosity into a household essential.
The Physics Behind the Magic: A Closer Look
To truly appreciate the vacuum flask, let's delve deeper into the physics at play:
The Role of the Vacuum
The vacuum in a thermos is crucial because it eliminates two major forms of heat transfer:
Conduction: In normal materials, heat is conducted when energetic molecules collide with less energetic ones, transferring energy. In a vacuum, there are almost no molecules to collide, so this transfer can't happen.
Convection: This requires a fluid (like air) to create currents that carry heat. No air means no convection.
The effectiveness of the vacuum can be quantified by its thermal conductivity. Air has a thermal conductivity of about 0.024 W/mK at room temperature. In contrast, the effective thermal conductivity of the vacuum in a high-quality thermos can be as low as 0.001 W/mK, making it about 24 times more effective at preventing heat transfer than still air.
The Power of Reflection
The reflective coating tackles the third form of heat transfer: radiation. Here's a more detailed look at how it works:
All objects emit infrared radiation based on their temperature, as described by the Stefan-Boltzmann law: E = σT^4, where E is the energy radiated, σ is the Stefan-Boltzmann constant, and T is the absolute temperature.
The reflective coating, typically silver or aluminum, can reflect up to 95% of this radiation.
For a hot beverage, this means that 95% of the heat trying to escape as radiation is bounced back into the liquid.
For a cold beverage, 95% of the ambient heat trying to enter the flask is reflected away.
This reflection is similar to how a mirror reflects visible light, but it's working with infrared light instead. The high reflectivity in the infrared spectrum is what makes these coatings so effective for thermal insulation.
Advancements and Variations in Vacuum Flask Technology
While the basic principle of the vacuum flask hasn't changed much since its invention, there have been several notable advancements:
Materials: Modern flasks often use stainless steel instead of glass for the inner container, making them more durable and less prone to breakage. Some high-end models use titanium for even better durability and lighter weight.
Vacuum Quality: Improvements in manufacturing have allowed for even better vacuums, enhancing insulation. Some modern thermoses can achieve vacuums as low as 10^-6 torr, compared to early models which might have only reached 10^-3 torr.
Stopper Design: More effective stoppers have been developed to further reduce heat transfer at the flask's neck. Some designs incorporate multiple layers of insulation and sealing mechanisms.
Size and Shape Variations: From tiny flasks for medications to large industrial-sized containers, the technology has been adapted for various uses. Some designs now include wide-mouth openings for easier filling and cleaning.
Smart Technology Integration: Some manufacturers are experimenting with adding temperature sensors and displays to vacuum flasks, allowing users to monitor the temperature of their beverages in real-time.
DIY Experiment: Demonstrating Vacuum Flask Principles
To better understand the principles behind a vacuum flask, here's a simple experiment you can do at home:
Materials needed:
- Two identical glass jars with lids
- Hot water
- Aluminum foil
- Insulating material (like bubble wrap or foam)
- Thermometer
Steps:
- Wrap one jar completely in aluminum foil (shiny side in).
- Wrap the foiled jar in insulating material.
- Fill both jars with hot water of the same temperature (around 90°C or 194°F).
- Close the lids and place them side by side.
- Measure the water temperature in each jar every 30 minutes for a few hours.
You'll notice that the water in the insulated, foil-wrapped jar stays warmer for longer, demonstrating how reflection and insulation work together in a vacuum flask. While this setup doesn't create a vacuum, it illustrates the principles of reducing heat transfer through reflection and insulation.
Environmental Impact and Sustainability of Vacuum Flasks
In our increasingly eco-conscious world, it's worth considering the environmental impact of vacuum flasks:
Positive Impact: By keeping beverages at the desired temperature for longer, vacuum flasks can reduce energy consumption associated with reheating or cooling drinks. A study by the International Journal of Consumer Studies found that using a reusable vacuum flask instead of disposable cups can reduce an individual's carbon footprint by up to 50% over a year.
Durability: Well-made vacuum flasks can last for years, reducing waste from disposable cups and bottles. A single high-quality thermos can replace hundreds or even thousands of disposable containers over its lifetime.
Materials: Many modern vacuum flasks are made from recyclable materials like stainless steel. At the end of their life, these materials can be reclaimed and repurposed.
Energy-Intensive Production: However, it's important to note that the production of vacuum flasks, particularly the process of creating the vacuum, can be energy-intensive. The environmental benefit comes from long-term use, offsetting the initial production impact.
Potential for Improvement: Ongoing research is exploring ways to make the production process more environmentally friendly, including using renewable energy in manufacturing and developing more sustainable materials for insulation.
The Future of Vacuum Flask Technology
While the basic principle of the vacuum flask is well-established, research continues in several exciting areas:
Improved Materials: Scientists are exploring new materials that could enhance insulation properties even further. For example, aerogels, which are ultra-light materials with excellent insulating properties, are being investigated for use in next-generation vacuum flasks.
Smart Technology Integration: Some manufacturers are experimenting with adding temperature sensors and displays to vacuum flasks. Future models might include smartphone connectivity, allowing users to monitor and control the temperature of their beverages remotely.
Nanomaterials: Research into nanomaterials could lead to even more effective insulation in smaller spaces. For instance, carbon nanotube-based coatings could provide superior reflectivity and durability compared to current metallic coatings.
Self-Heating and Cooling: Some researchers are working on integrating thermoelectric elements into vacuum flasks, which could allow for active heating or cooling of the contents using the Peltier effect.
Sustainable Production: Efforts are being made to make the production process more environmentally friendly, including using renewable energy in manufacturing and developing more sustainable vacuum-creation methods.
Advancements in Vacuum Technology: Ongoing research in creating and maintaining higher-quality vacuums could lead to even more effective insulation in future vacuum flasks.
Conclusion: The Enduring Legacy of a Simple Idea
The vacuum flask stands as a testament to the power of simple, elegant scientific principles applied to everyday problems. From keeping our coffee hot on the morning commute to enabling critical scientific research, this invention has become an indispensable part of modern life.
As we've explored, the magic of the thermos lies in its ability to create a formidable barrier against all forms of heat transfer – conduction, convection, and radiation. By understanding these principles, we gain not just an appreciation for this humble device, but also insight into fundamental concepts of thermal physics.
The vacuum flask's impact extends far beyond personal convenience. Its technology has found applications in scientific research, medical transport, space exploration, and industrial processes. It has contributed to energy conservation efforts and plays a role in our ongoing quest for sustainability.
The next time you sip a hot drink from your thermos on a cold day, or enjoy a cool beverage in the summer heat, take a moment to appreciate the ingenious science at work. It's a daily reminder of how understanding and harnessing the laws of nature can lead to practical, life-enhancing technologies.
In a world of rapid technological change, the enduring usefulness of the vacuum flask stands as a testament to the timeless value of good design based on sound scientific principles. It's a simple solution that continues to serve us well, more than a century after its invention, and promises to evolve and improve as we push the boundaries of materials science and engineering.
The story of the vacuum flask is far from over. As we continue to innovate and refine this technology, we can look forward to even more efficient, sustainable, and versatile thermal containers in the future. The principles that make the thermos work – the manipulation of heat transfer mechanisms – will undoubtedly play a crucial role in addressing larger challenges, from energy conservation to space exploration.
In essence, the humble thermos is not just a container for our beverages – it's a capsule of scientific ingenuity, a testament to human creativity, and a daily companion in our lives. It reminds us that sometimes, the most profound solutions are also the simplest, and that understanding the fundamental laws of nature can lead to innovations that stand the test of time.