Introduction: Illuminating the Mystery
For thousands of years, candles have cast their warm glow across human civilization, serving as beacons of light, symbols of celebration, and focal points for contemplation. Yet, beyond their flickering beauty lies a fascinating world of chemistry and physics. This article delves deep into the science of candle combustion, with a particular focus on the gases produced when a candle burns. As we embark on this illuminating journey, we'll uncover the intricate processes that occur within that dancing flame, exploring not just what gases are produced, but how and why they form.
The Fundamentals of Candle Combustion
At its core, a candle is a simple yet ingenious device that harnesses the process of combustion to produce light and heat. To understand the gases produced, we must first grasp the basics of how a candle functions.
The Anatomy of a Candle
A typical candle consists of three main components:
- The wax: Usually paraffin, soy, or beeswax, this serves as the fuel source.
- The wick: A braided cotton string that draws up the melted wax.
- The flame: The visible manifestation of the combustion process.
When a candle is lit, the heat from the flame melts the nearby wax. This liquid wax is then drawn up the wick through capillary action. As it reaches the flame, the liquid wax vaporizes and undergoes a chemical reaction with oxygen in the air – this is combustion.
The Primary Gas Produced: Carbon Dioxide
The Result of Complete Combustion
In an ideal scenario, where a candle burns with perfect efficiency, the primary gas produced is carbon dioxide (CO2). This colorless, odorless gas is a natural byproduct of the combustion of hydrocarbons, which is exactly what most candle waxes are composed of.
The Chemistry Behind CO2 Production
To understand why carbon dioxide is the main gas produced, let's look at the chemical composition of candle wax. Most waxes are long chains of hydrocarbons, typically with the formula CnH2n+2. When these hydrocarbons burn completely in oxygen, the carbon atoms combine with oxygen to form CO2, while the hydrogen atoms combine with oxygen to form water vapor (H2O).
The general equation for this reaction can be written as:
CnH2n+2 + (3n+1)O2 → nCO2 + (n+1)H2O
For example, if we consider a simple hydrocarbon like octane (C8H18), which is similar to some components in candle wax, the equation would be:
C8H18 + 12.5O2 → 8CO2 + 9H2O
This reaction illustrates why carbon dioxide is the primary gas produced in candle combustion.
Quantifying CO2 Production
The amount of CO2 produced by a burning candle is relatively small compared to many other sources. According to a study published in the journal "Science of the Total Environment," a typical paraffin candle burning for one hour produces about 10 grams of CO2. To put this in perspective, this is roughly equivalent to the CO2 produced by leaving a 60-watt incandescent light bulb on for 15 minutes.
Water Vapor: The Often Overlooked Byproduct
While carbon dioxide often takes center stage in discussions about combustion products, water vapor is an equally important byproduct of candle burning.
The Formation of H2O
As seen in the chemical equation above, the hydrogen atoms from the wax combine with oxygen from the air to form water (H2O). This water is initially in the form of steam due to the high temperatures in and around the flame.
Observing Water Production
You can actually observe this water production through a simple experiment:
- Light a candle and allow it to burn steadily for a few minutes.
- Carefully hold a cold, dry glass or mirror above the flame (at a safe distance to avoid cracking or overheating).
- Within seconds, you'll notice condensation forming on the surface of the glass or mirror.
This condensation is the water vapor produced by the candle, cooling and returning to its liquid state upon contact with the cold surface.
Beyond CO2 and H2O: Other Gases and Particles
While carbon dioxide and water vapor are the primary products of ideal candle combustion, real-world burning is rarely perfect. Several other substances can be produced, especially under suboptimal burning conditions.
Carbon Monoxide: The Danger of Incomplete Combustion
When there's insufficient oxygen for complete combustion, carbon monoxide (CO) can form instead of carbon dioxide. This colorless, odorless gas is highly toxic, binding to hemoglobin in the blood and reducing oxygen transport.
Fortunately, under normal conditions with proper ventilation, the amount of CO produced by candles is minimal. A study published in the journal "Indoor Air" found that even multiple burning candles in a poorly ventilated room produced CO levels well below safety thresholds.
Soot and Particulate Matter
Candles can also produce small particles of soot, which is essentially unburned carbon. These tiny particles can become airborne and may be inhaled. The production of soot is often visible as black smoke when a candle burns improperly, such as in a draft or with an overly long wick.
A 2001 study by the U.S. Environmental Protection Agency found that candles with metal-core wicks may emit lead into the air along with the soot, though such wicks are now rare in quality candles.
Volatile Organic Compounds (VOCs)
Some candles, particularly scented varieties, may release various volatile organic compounds. These can come from both the wax itself and any added fragrances. A 2009 study published in "Environmental Science & Technology" detected over 300 different VOCs in the emissions from scented candles, including some known carcinogens like formaldehyde and acetaldehyde.
However, it's important to note that the concentrations of these VOCs were generally very low, and occasional use of scented candles in well-ventilated areas is unlikely to pose significant health risks for most people.
Factors Influencing Gas Production in Candles
Several variables can affect the types and quantities of gases produced by a burning candle:
Wax Composition
Different types of wax can produce slightly different combustion products. For example:
- Paraffin wax, derived from petroleum, tends to produce more soot and potentially more VOCs.
- Soy wax, made from soybean oil, generally burns cleaner with less soot.
- Beeswax is often considered the cleanest burning, producing minimal soot and naturally occurring VOCs.
Wick Characteristics
The size, material, and condition of the wick play crucial roles in combustion efficiency:
- A properly sized wick ensures steady fuel supply and complete combustion.
- Cotton wicks are most common and generally burn cleanly.
- An untrimmed wick can lead to a larger, less stable flame, increasing soot production.
Environmental Factors
The surrounding environment significantly impacts candle combustion:
- Air flow: Good ventilation provides enough oxygen for cleaner burning but can also cause uneven burning if too strong.
- Altitude: At higher altitudes, the lower oxygen concentration can affect combustion efficiency.
- Humidity: High humidity can make it slightly harder for a candle to stay lit and may affect the burning rate.
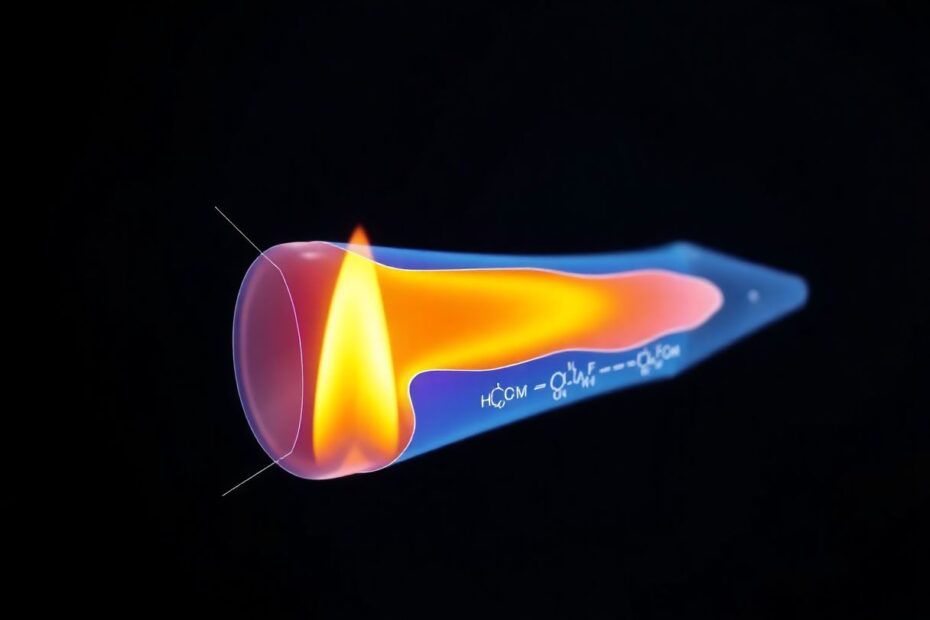
The Complexities of Candle Flame Structure
A closer look at the candle flame reveals a complex structure with distinct zones, each characterized by different temperatures and chemical processes.
Zones of the Flame
The Blue Zone: Located at the base of the flame, this is where oxygen is most abundant. Here, combustion is nearly complete, producing primarily CO2 and H2O.
The Dark Zone: Just above the wick, this region contains vaporized wax that hasn't yet combusted. It's cooler than the surrounding flame.
The Yellow Zone: This is the bright, visible part of the flame where most light is produced. Here, carbon particles become incandescent before fully combusting.
The Outer Veil: A faint, nearly invisible layer surrounding the flame where final combustion occurs.
Temperature Variations
The temperature within a candle flame varies dramatically:
- The hottest part, reaching up to 1400°C (2552°F), is typically in the pale blue outer edges of the flame.
- The coolest part is near the wick, where temperatures are around 600-800°C (1112-1472°F).
These temperature variations play a crucial role in the types and amounts of gases produced at different points in the flame.
Environmental and Health Implications
Understanding the gases produced by candles is crucial for assessing their environmental and health impacts.
Indoor Air Quality
While occasional candle use is generally safe, frequent burning in poorly ventilated spaces can impact indoor air quality. The primary concerns are:
- Particulate matter (soot) which can irritate respiratory systems
- VOCs, especially from scented candles, which may cause headaches or irritation in sensitive individuals
- CO2 buildup in very poorly ventilated spaces, though this is rare under normal conditions
To minimize potential issues:
- Ensure good ventilation when burning candles
- Choose candles made from natural waxes like soy or beeswax
- Opt for unscented candles if you're sensitive to fragrances
- Keep wicks trimmed to about 1/4 inch to reduce soot production
Carbon Footprint Considerations
The carbon dioxide produced by candles is minimal compared to many other household activities. However, for the environmentally conscious:
- Consider candles made from renewable sources like soy or beeswax
- Use candles mindfully, not excessively
- LED alternatives can be considered for purely functional lighting needs
Candles in Scientific Research and Education
The simplicity and accessibility of candles make them excellent tools for scientific research and education.
Classroom Experiments
Candles offer numerous opportunities for hands-on learning:
- Observing combustion reactions in real-time
- Studying heat transfer through conduction, convection, and radiation
- Exploring gas properties and detection methods
Historical Significance in Science
The study of candle flames has played a pivotal role in the development of combustion science. Michael Faraday's famous series of lectures, "The Chemical History of a Candle," delivered in 1848, demonstrated how much fundamental science could be learned from these simple devices. Faraday used candles to explain concepts in chemistry, physics, and engineering, many of which remain relevant today.
Modern Applications of Candle Science
Research into candle combustion continues to have relevance beyond simple illumination:
Advanced Scent Delivery Systems
Understanding how candles vaporize and release compounds has led to the development of sophisticated scent delivery systems used in aromatherapy, home fragrancing, and even in some forms of medical treatment.
Materials Science and Engineering
Ongoing research into wick materials, wax formulations, and combustion dynamics drives improvements in candle performance, longevity, and reduced emissions. This research often has applications in other fields, such as fuel science and thermal management.
Fire Safety and Prevention
Studying candle combustion helps in developing better fire prevention strategies and firefighting techniques. Understanding how different materials burn and what gases they produce is crucial for fire safety engineering.
Conclusion: A Flickering Window into Chemistry
From the primary production of carbon dioxide and water vapor to the complexities of flame structure and the nuances of different wax types, the humble candle offers a fascinating glimpse into the world of chemistry and physics. What appears as a simple flame is, in reality, a sophisticated interplay of heat, fuel, and oxygen, producing a range of gases and particles.
As we've explored, the main gases produced by a burning candle – carbon dioxide and water vapor – are complemented by a host of other substances depending on various factors. This complexity serves as a reminder of the intricate chemical processes occurring in even the most commonplace objects around us.
Whether you're lighting a candle for ambiance, using it in scientific study, or simply enjoying its warm glow, you're participating in a tradition that spans millennia and continues to illuminate our understanding of the natural world. So the next time you light a candle, take a moment to appreciate the miniature chemical factory you've just set in motion – a testament to the endless fascination found in everyday science.